
The science behind spectacular Canada Day firework displays
Green peonies, blue dahlias, red crossettes, white and silver chrysanthemums; how are all of these incredible firework displays produced?
Canada Day is coming up and numerous fireworks shows will no doubt be occurring across the country. Here’s the amazing science behind these delightful displays.
On the night of Tuesday, July 1, 2025, Canadians will celebrate the 158th anniversary of the unification of our country. Formerly known as Dominion Day, this celebration often includes the lighting of fireworks — from small backyard displays up to immense spectacles that light up entire city skylines.
Regardless of their size, all fireworks are formed from the combination of two basic components — the sparkler and the firecracker.
The sparkler
In the time leading up to the start of a fireworks display, we can already see some of the science at work as children of all ages light and wave sparklers around in the air.

A lit sparkler. (Jiří Wagner (Unsplash))
The characteristic sparks thrown off by these seemingly magical wands result from burning a very specific mixture of chemicals and powders. The mixture will always contain three specific components — 1) some kind of powdered metal, 2) a chemical oxidizer, and 3) some kind of starchy substance to bind everything together and make it stick to the metal handle. Sometimes charcoal or sulfur are added to the mixture, providing extra fuel for the sparkler to burn.
For the sparkler to work, this combination of substances has to be very carefully controlled.
"In order to produce an intense glow, more oxygen is needed than is available from the surrounding air," says Dr. Joe Schwarcz, the director of McGill University's Office for Science and Society. "This is where the potassium perchlorate comes in. It is an oxidizing agent, meaning that it is a source of oxygen. Potassium chlorate undergoes a chemical reaction, initiated by the lighting of the sparkler, whereby it decomposes to yield potassium chloride and oxygen. The oxygen then combines with the metal, allowing it to burn. This combustion process produces heat, which then decomposes more potassium perchlorate, yielding more oxygen, which then combines with more metal, and on and on until one of the reagents runs out."
Even though sparklers don't explode, anyone holding one — especially children — should be very careful! The point on the wand emitting the sparks reaches temperatures of at least 1,000°C, which is more than enough to cause severe burns or even set clothing on fire.
Also, be mindful of where the sparks land, especially if there has been a stretch of dry weather in the area.
The firecracker
Firecrackers also work based on the combination of three different chemical components. However, rather than providing minutes of enjoyment with bright, flying sparks, they have but one purpose — to explode.

A bundle of unlit firecrackers. Credit: Ritesh Man Tamrakar/Flickr (CC BY 2.0)
The primary chemical component of a firecracker is gunpowder, which consists of a very specific combination of sulfur, charcoal, and potassium nitrate. When burned, carbon from the charcoal reacts with the sulfur and potassium nitrate, releasing nitrogen and carbon dioxide gases, along with plenty of heat.
To make a firecracker, a paper tube or sphere is packed with enough gunpowder to fill it. A fuse made of paper or fabric wrapped around a gunpowder core is added to deliver an ignition flame to the inside of the container. The entire thing is then sealed, air tight.
Once the fuse is lit, the flame travels along until it reaches and ignites the gunpowder. In a fraction of a second, the expanding nitrogen and carbon dioxide exert tremendous pressure on the interior surface of the container, causing it to burst open. The explosion sets off a wave of compressed air that expands in all directions, which we hear as a bang when it passes our ears.
The only difference between firecrackers, really, is the size. A larger firecracker means there is more gunpowder packed within it, thus resulting in a larger and louder explosion.
DON'T MISS: The Weather Network's new Fire & Smoke map helps you plan ahead and stay safe
The Big Show
The immense and spectacular firework displays we see on holidays like Canada Day result from combining sparklers and firecrackers to produce something called a firework shell.

Some firework shells can be MUCH more complex, like this roughly metre-wide Japanese shell, which features not only sparkler spheres lining the interior but also nested shells that contain even more sparkler spheres, designed to go off well after the initial burst. Credit: Joel(frikitiki)/Flickr (CC BY-ND 2.0)
The most basic firework shell can be thought of as a nested, up-scaled firecracker. A small firecracker known as a burst charge is placed at the centre of a large hollow paper tube or sphere, along with a timing fuse linking it directly to the outside of the shell. Surrounding it, filling in all the extra space in the shell, is a mixture of gunpowder and tiny sparkler spheres called 'stars'.
After the timing fuse is lit, the shell is fired into the air from a special launch tube, sometimes using a launch charge incorporated directly into the bottom of the shell. The timing fuse is slow-burning compared to the rest of the firework components, and determines the exact height above the ground where the firework shell explodes.
When the burst charge detonates, the gunpowder ignites, simultaneously lighting the sparkler stars and blowing the shell apart, scattering the stars in all directions.

A multi-colour firework burst. Credit: DeltaWorks/Pixabay
The colours of the resulting pyrotechnic display depend on the exact makeup of the sparkler stars.
Some of the most common colours, and their corresponding chemicals, are:
White — aluminum or magnesium
Silver — aluminum, magnesium, or titanium powder
Blue — copper
Red — strontium or lithium
Green — barium
Yellow — sodium
Orange — calcium
Purple — strontium and copper

The different metals or chemical compounds that produce the different firework colours. Credit: Base firework image by Artur Strecker/Pixabay, infographic by Scott Sutherland
The coloured light results from a process similar to what we see from both fluorescent lightbulbs and the Aurora Borealis.
In their normal, 'ground' state, each atomic element — aluminum, strontium, copper, etc. — has a different number of electrons orbiting around the nucleus, arranged in what scientists call 'shells'. When the atom absorbs energy, such as when you apply heat, one or more of those electrons 'jump up’ to orbit farther away from the nucleus. This is called the atom's 'excited' state, and each atom requires a different amount of energy for this ‘jump’ to occur.
To return to its normal orbit (the ‘ground state’), the electron has to dump that excess energy somehow. It does so by releasing a photon of light, and when it comes to photons, energy = colour.
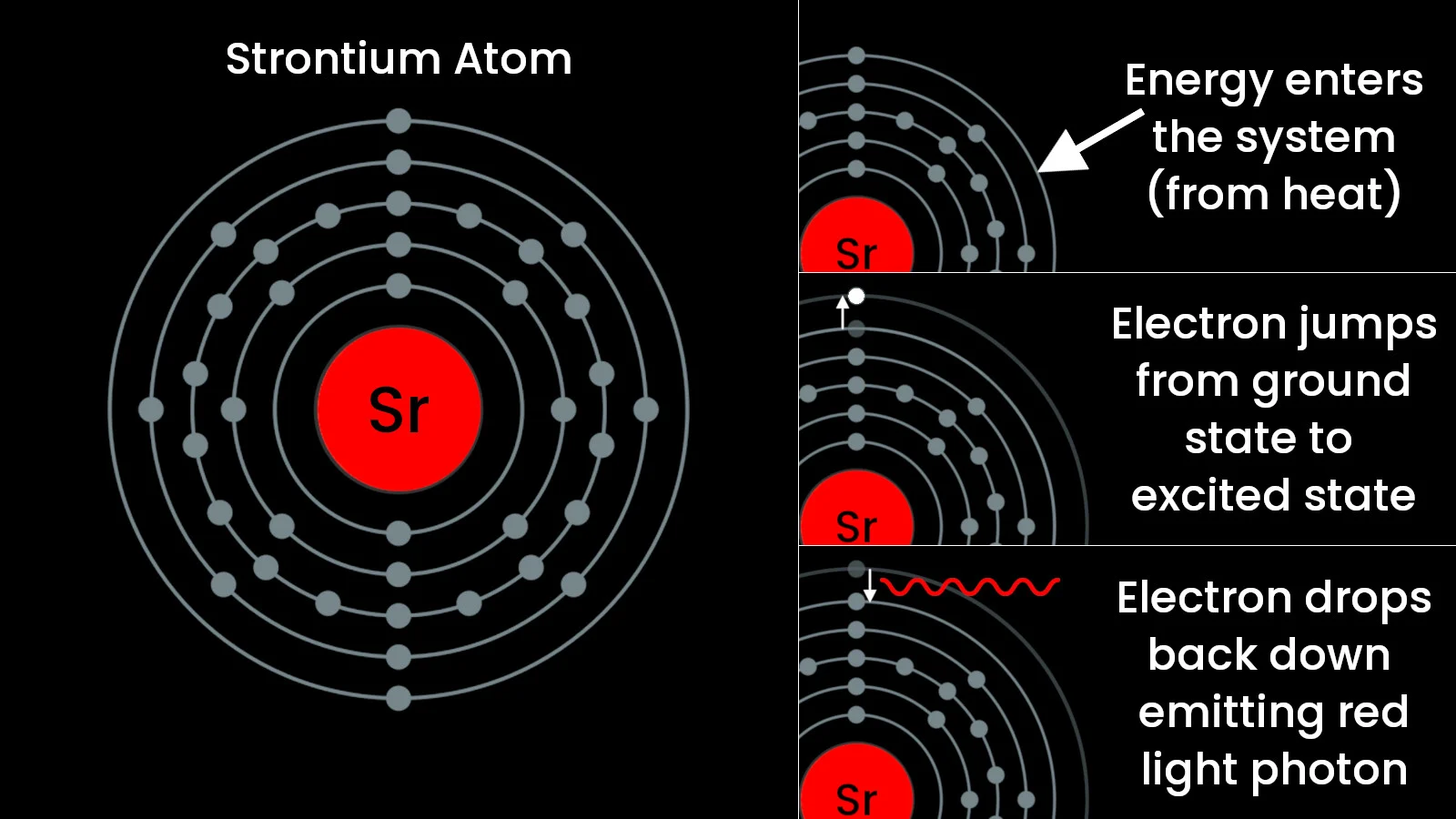
The process by which a strontium atom emits red light. (Electron shell diagram by Pumbaa/Greg Robson/Wikimedia (CC BY-SA 4.0), infographic by Scott Sutherland)
Lower energy gives you a slower oscillating photon, which puts the colour more towards the red end of the spectrum. Higher energy produces faster oscillation, which makes the photon more blue.
This is all a bit of an oversimplification, of course. As shown in the image above, these atoms have multiple levels (also called 'shells') of electrons, and multiple electrons can be jumping up to higher levels and then dropping back down as the atoms are energized by the burning process, emitting different wavelengths of light as they do. The wavelength of light with the greatest number of photons being emitted is the colour that we see from all of this.
Elements like aluminum, magnesium, and titanium are special cases. While the others mentioned here mainly emit one wavelength of light, these atoms emit light across nearly the entire visible spectrum. So, just like with sunlight, all the colours they emit blend together to make white light. In this same way, elements can be combined in different quantities to delve into other colours, too, such as mixing strontium and copper to get red and blue light at the same time, resulting in purple.
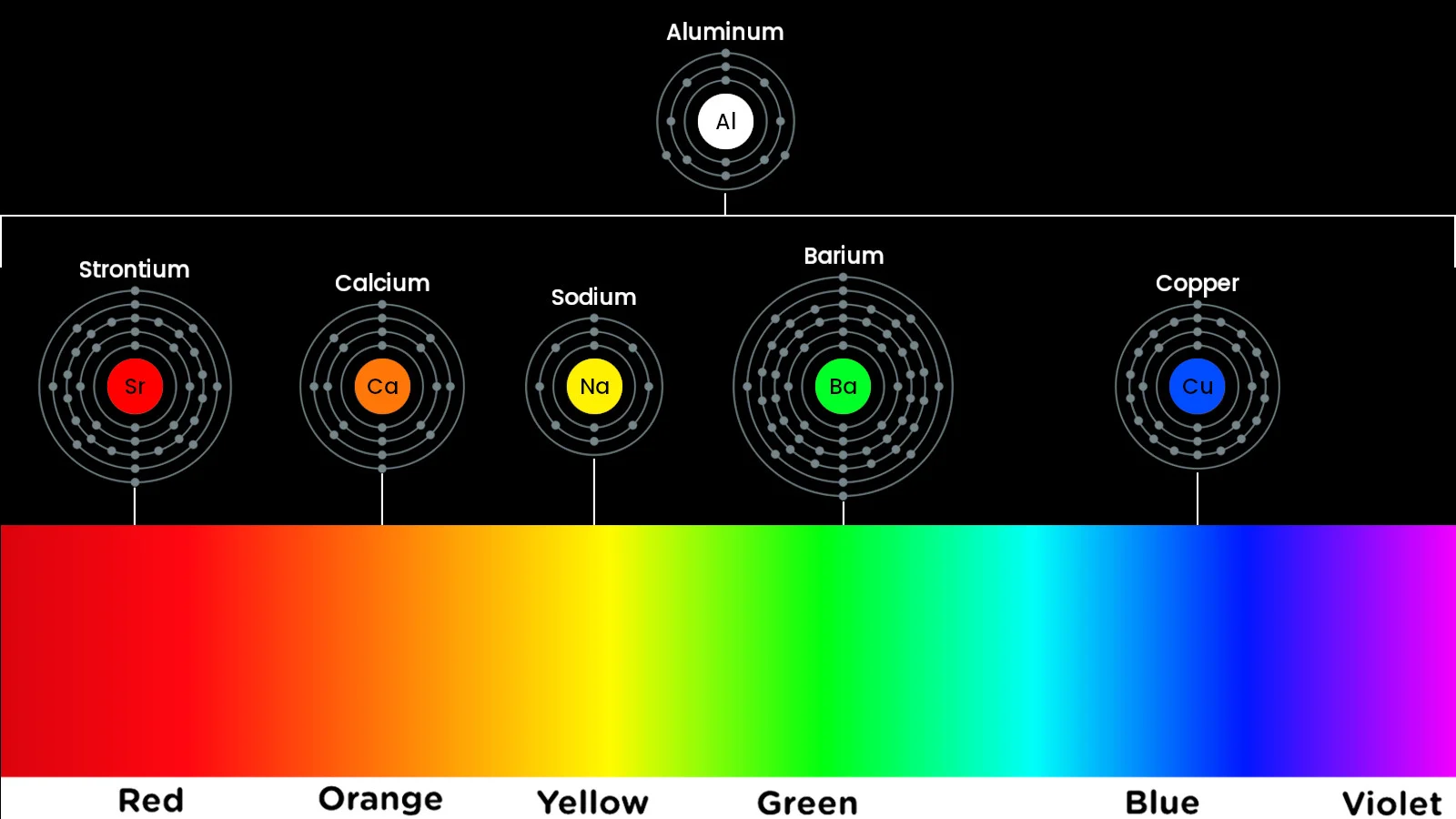
The visible spectrum of light and the colours produced by the different elements used in fireworks. (Image by Scott Sutherland, colour spectrum by Meganbeckett27/Wikimedia (CC BY-SA 3.0), atomic shell diagrams by Pumbaa/Greg Robson/Wikimedia (CC BY-SA 4.0))
As for the shape produced by a specific firework, this is due to the stars' precise arrangement within the shell.
When the stars are packed into a firework shell randomly, it results in a spherical display known as a peony, named after the flower.
More careful arrangements of stars, along with using stars with different chemical mixtures, allows for much more specific shapes and designs. This is how we get other flower-like displays, such as the dahlia, chrysanthemum, and pistil.

This composite image shows (left to right) peony, chrysanthemum, dahlia, and pistil displays. Credits: Images by Tuan Hung Nguyen/Pixabay, composite by Scott Sutherland
Tree-like palm and willow displays are also popular, and patterns can be arranged to produce rings, stars, spiders, waterfalls, and a host of other shapes as well.
Special effects can be achieved by organizing layers of gunpowder and stars that go off at different times. One way of accomplishing this is by packing a larger shell with several smaller shells. Another way is to change the composition of the stars so that they burn longer, or crackle and whistle as they burn. They can even coat tiny firecrackers with sparkler material to produce fiery trails that end with small explosions.
The combinations are quite endless, limited only by the imagination of the designer!

This New Year's fireworks display shows off the variety of possible patterns and colours. Credit: Nick/Pixabay
And the really great thing is that knowing the science that goes into making these fantastic displays takes absolutely nothing away from the awe they inspire!
